PLEASE READ THE FOLLOWING SAFETY NOTICES
LASER SAFETY
The Kernel Flow complies with Federal Laser Product Performance Standards (FLPPS) 21CFR1040.10 (Code of Federal Regulations, Title 21, Section 1040.10) and is classified as a Class 1 laser device and does not present a hazard with regard to maximum permissible exposure and accessible exposure limits for both ocular and skin considerations.
The Kern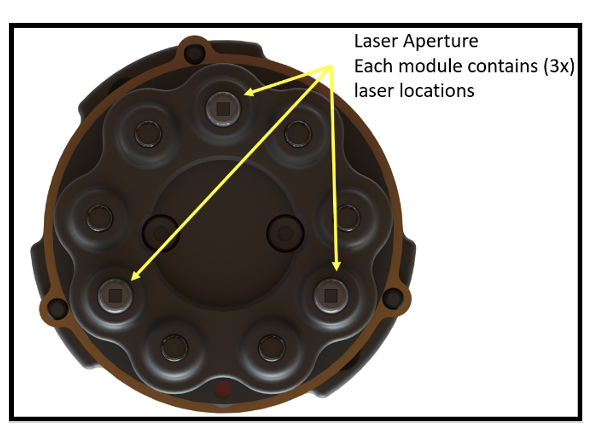 el Flow utilizes 120 NIR and 120 visible Class 1 diode lasers. During Normal operation the Kernel Flow emits visible (690nm) and invisible (905nm) Class 1 laser radiation.
el Flow utilizes 120 NIR and 120 visible Class 1 diode lasers. During Normal operation the Kernel Flow emits visible (690nm) and invisible (905nm) Class 1 laser radiation.Class 1 laser is considered safe from all potential hazards and is considered to be incapable of producing damaging radiation levels during operation and is exempt from any control measures.
Natural eye protection is provided by the physiological eye aversion response. This response induces closure of the eyelid, eye movement and pupillary constriction, in addition to movement of the head to avoid an exposure to a bright light stimulant. The aversion response to an exposure from a bright, visible, laser source is assumed to limit the exposure of a specific retinal area to 0.25 s or less.
The Kernel Flow is designed to be positioned on the user prior to activation. Users should not intentionally stare into or at the source lasers.
CAUTION
DEVICE IS NOT USER-SERVICEABLE
Any misuse or mishandling of the product, including failure to properly clean and store the product, will void the warranty.
Kernel Flow has not been evaluated for clinical performance or use, accuracy, reliability, or effectiveness, and has not been validated for any particular use.
FOR RESEARCH USE ONLY
Kernel Flow is for research use only and has not been tested for electrical safety, electromagnetic compatibility, or biocompatibility.
NOTE:
.png)
Per 21CFR1010.3 you will find this label on the card included with your Flow system stating the month of manufacture. This information is additionally available inside the hub enclosure of the Flow headset itself.